
Since ancient times it has been described that seizures present a cyclical organization. Despite this, only with the wider use of the long-term electroencephalography (or EEG for short) clinicians and scientists have been able to further identify and classify the seizure patterns. We now know that seizures may happen only at certain times of the day (and/or night) which is called a circadian rhythm; or at certain times of the month or even the year, called multidien and circannual rhythms, respectively.
Many clinical studies have been addressing the complex cyclical structure of epilepsy – which is very exciting, especially because all these changes over time could impact diagnosis and treatment. However, we still have a journey ahead in order to understand what is happening in our brain cells that is able to drive these epilepsy cycles. In other words, we need to fully understand how the epilepsy clocks are ticking!
For that, let’s zoom in and check our brain’s clock first, which is called the suprachiasmatic nucleus (SCN). Yes, our brains have a central clock (in the case you were wondering). The SCN is the tiny structure you can see marked in green in the figure below.
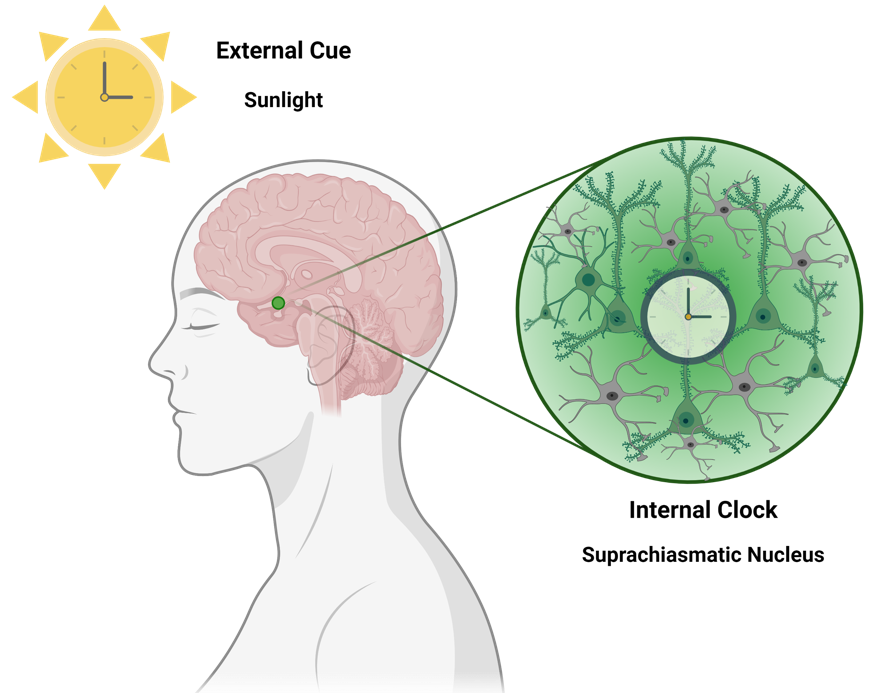
The majority of the neurons from the SCN (about 78% of them, as reported by Dr Elise Drouyer) respond to light exposure, which is able to increase their activity. This light is from the “outside world” – it is the light that our eyes can capture and the retina in the eye ‘informs’ the neurons about it. Thus, the presence or absence of light are very relevant to ‘adjust our brain clock’. However, this is not the only source of regulation – the neurons in the SCN also have their own individual ‘internal clocks’. In fairness, all cells of our body have their own internal clocks – but let’s not get overwhelmed (we can chat about it another time!). Back to the neurons, there are some genes (the most famous ones are Bmal1, Clock, Cry and Per) which cycle in harmony and regulate each other (similar to an automatic watch). Their full regulatory cycle takes 24 hours (= circadian). These genes are the main players responsible for keeping our circadian rhythms in sync.
How are our clocks in epilepsy?
We are just starting to understand in detail the internal clocks of our brain cells in epilepsy. Moreover, circadian scientists discovered that the internal molecular clocks of the cells are much more complex than we initially thought – and a much higher number of genes belong to that group of ‘core clock genes’. This adds extra layers of complexity to the whole process. Among these important discoveries is the work from Dr Tianpeng Zhang, from the Institute of Molecular Rhythm and Metabolism (Guangzhou University of Chinese Medicine) recently published in Nature Communications. In this work, Dr. Zhang and collaborators investigated the role of a gene called REV-ERBα (I know, so difficult to pronounce!) in the circadian regulation of epileptic seizures. This gene is known to regulate the main molecular clock (remember: Bmal1-Clock; Cry-Per). I said it would become complex, so let’s simplify it.
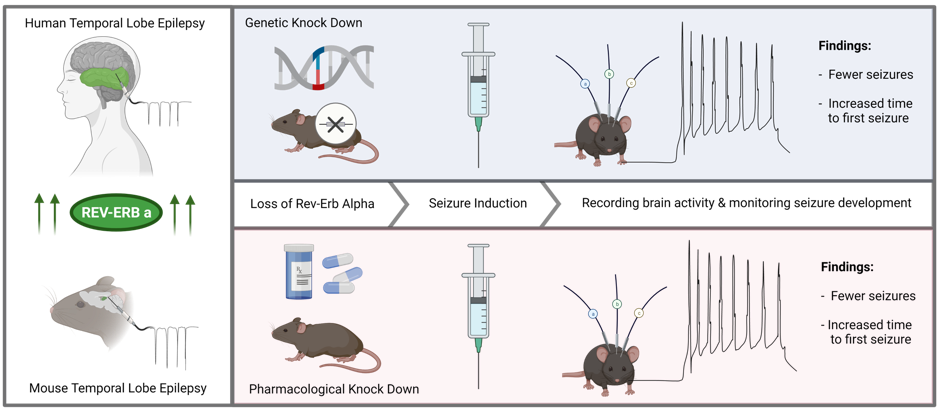
Dr. Zhang’s and colleagues first looked at the levels of REV-ERBα in brain tissues from people with epilepsy and in an experimental model that mimics seizures. They found that the levels were increased, showing a connection between seizures and the gene. Next, they used a transgenic mouse model which didn’t have the Rev-ERBα gene at all (we call them ‘knockouts’ or KO) to understand how these mice would develop seizures. For this, the KO mice were injected with a chemoconvulsant called kainic acid (which induces seizures in mice after a single injection) and were observed on EEG. In this experiment they found that it not only took longer for the Rev-ERBα KO mice have their first seizure after the kainic acid injection, but the severity of the seizure was also reduced. In order to confirm these findings they used a different cohort of mice – at this time mice without any genetic modification. They treated these mice with a small molecule called SR8278, which is able to pharmacologically inhibit Rev-ERBα (to test it as a possible medicine), and after the treatment, they again induced seizures by the injection of kainic acid. This experiment only mimicked status epilepticus, thus, to increase relevance they’ve also used a different mouse model that mimics temporal lobe epilepsy, called pilocarpine model, which allowed them to look at the effect in recurrent seizures. Overall, they concluded that decreasing the levels of Rev-ERBα (either using KO mice or via treatment) decreases the susceptibility to acute and chronic seizures (take a look at the figure below). On the other hand, activation of Rev-ERBα increases seizures in mice.
How does it happen?
In order to establish the possible mechanisms around this protective effect of the Rev-ERBα reduction, they looked at individual cells and verified that after the treatment there was an increase in GABAergic signalling – which is the major inhibitory circuitry in the brain. Interestingly, they’ve identified that Rev-ERBα promotes the expression of genes called Slc6a1 and Slc6a11, which are responsible for clearance of GABA. Thus, less Rev-ERBα indirectly results in more GABA available (= reduction of brain hyperexcitability).
What’s next?
Although these findings are very exciting and bring further understanding of how the brain clocks are (dys)regulated in epilepsy, we still have a lot more to discover. The brain cells clocks are for sure the most complex automatic watches ever! Moreover, we still don’t know which is the ‘main player’ or which gene is the master-regulator of all the ongoing processes. But still, it is very exciting to find out that regulating the circadian clock may be a novel therapeutic approach for epilepsy. Until then, the best (and adjuvant) approach is to keep our rhythms in sync… and for sure healthy routines and a good night sleep help!
About the author – Cristina Reschke

Cristina is Brazilian neuroscientist who adopted Dublin as home. She studied Pharmacy and worked in hospitals for three years when she decided to follow her childhood dreams – becoming a scientist. In Brazil, Cristina did a Masters and a PhD in Pharmacology focused on brain inflammation and epilepsy and, as part of her training, spent about a year in Italy. She moved to Ireland in 2013 for her postdoctoral training at RCSI and in late 2020 she was appointed Lecturer in the School of Pharmacy and Biomolecular Sciences. Her team is interested in investigating the molecular mechanisms of epilepsy development in order to develop better treatments – specifically, how disruptions of circadian rhythms affect gene expression. In her free time, Cristina enjoys walks and hikes, gardening (and staying in contact with nature), listening to audiobooks and a good chats with friends – not necessarily in this order. Cristina is co-founder of Epilepsy in English with Gareth.

Be the first to comment